A gigantic freshwater aquifer is hiding under the salty Atlantic Ocean, just off the northeastern coast of the United States.
While the aquifer’s exact size is still a mystery, it may be the largest of its kind, taking up a region stretching from at least Massachusetts to southern New Jersey, or nearly 220 miles (350 kilometers).
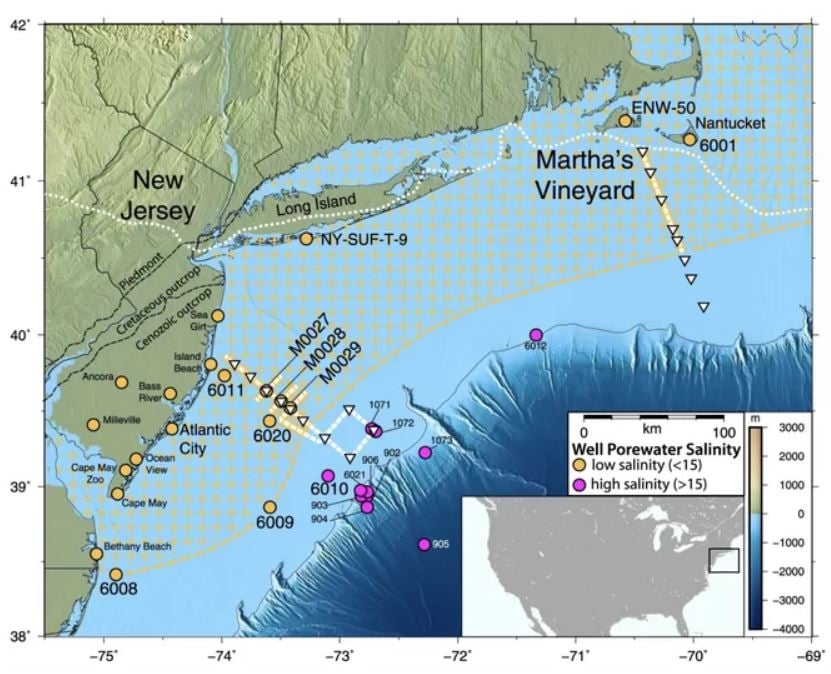
The area includes the coastlines of New York, Connecticut and Rhode Island. This aquifer may contain about 670 cubic miles (2,800 cubic kilometers) of slightly salty water.
This water isn’t young, either. The researchers said they suspect that much of it is from the last ice age.
These days, it appears that the aquifer isn’t stagnant. Rather, it’s likely fed by subterranean runoff from the land, the researchers said. This water is then likely pumped seaward by the rising and falling pressure of the tides.
Mysterious aquifer
Scientists got the first hints that an aquifer was hanging out under the ocean in the 1970s, when companies drilling off the coast for oil sometimes hit freshwater instead. But it wasn’t clear whether these freshwater water deposits were isolated pockets or whether they covered a larger expanse.
About 20 years ago, study co-researcher Kerry Key began helping oil companies pinpoint oil hotspots by using electromagnetic imaging on the subseafloor. Now using this same technology, he decided to find new aquifers – underground pools of fresh water. So, choosing spots were oil companies had reported finding fresh water, he started to look for freshwater under the Atlantic Ocean.
“We knew there was fresh water down there in isolated places, but we did not know the extent or geometry,” said lead author Chloe Gustafson.
First analyses… unprecedented results
To investigate these areas, the researchers dropped instruments to the seafloor to measure the electromagnetic fields below. In addition, a tool towed behind the ship emitted artificial electromagnetic pulses and measured the reactions from the subseafloor. The idea between the two methods being: Salt water conducts electromagnetic waves better than fresh water does, so any pools of fresh water would stand out as bands of low conductance.
An analysis found that the fresh water wasn’t scattered here and there, but was instead continuous, starting at the shoreline and extending out on the continental shelf. In some places, the aquifer stretched as far as 75 miles (120 km) offshore.
The feature also ran deep, starting at about 600 feet (182 meters) below the ocean’s floor and ending at about 1,200 feet (365 m) below the seafloor.
If later research shows that the aquifer is larger, it could rival the Ogallala Aquifer, a huge freshwater pool that supplies groundwater to eight Great Plains states, from South Dakota to Texas.
How freshwater ends up under the ocean?
The aquifer likely came into being at the end of the last ice age, the researchers said.
About 20,000 to 15,000 years ago, much of the world’s water was locked up in glaciers, making sea levels lower than they are now.
As temperatures rose and the ice covering the U.S. Northeast melted, water washed away huge quantities of sediments, which formed river deltas on the still-exposed continental shelf.
Large pockets of fresh water from the melted glaciers then got stuck in these sediment traps. Later, sea levels rose, trapping the sediment and fresh water under the ocean.

Slightly salty water
He added that the aquifer is freshest close to shore and gets saltier farther out, indicating that it slowly mixes with seawater over time.
The freshwater near land is about 1-part-per-thousand salt, much like other terrestrial fresh water. In contrast, by the aquifer’s outer edges, it’s about 15 parts per thousand, which is still lower than typical seawater’s level of 35 parts per thousand.
In other words, this water would have to be desalinated before people could use it, but it would still be cheaper to process than regular salt water.
Such large slightly salty aquifers in dry places such as Southern California, Australia, the Mideast or Saharan Africa might potentially represent a resource.
[Nature, Columbia.edu, LiveScience]












